Which Statement Describes a Hydrogen Bond Between Two Water Molecules
Which of the following statements describe hydrogen bonding between water molecules or describe properties of water that lead to hydrogen bonding between water molecules. The diagram below shows hydrogen bonds between water molecules.

Hydrogen Bonding Chemistry For Non Majors
1 How the bond between two oxygen atoms is formed.

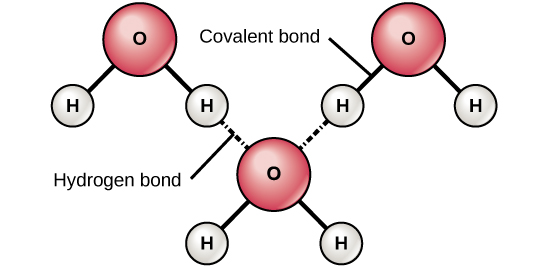
. Hydrogen bonding is an attractive force between two molecules that relies on the slight polarity of the O-H O-F or O-N bond. What describes each hydrogen bond. Hydrogen bonding forms in liquid water as the hydrogen atoms of one water molecule are attracted towards the oxygen atom of a neighboring water molecule.
5 How would you describe the bond that exists between two oxygen atoms to make. An additional two bonds can be formed between each hydrogen atom and nearby oxygen atoms. An attraction between neutral ends of two molecules.
A weak bond in which the oxygen atom of one molecule takes an electron away from the hydrogen atom of another water molecule a weak bond between the slightly negative oxygen atom of one water molecule and the slightly positive hydrogen atoms of another water molecule a weak bond. The hydrogen bond in water is a dynamic attraction between neighboring water molecules involving one hydrogen atom located between the two oxygen atoms. An attraction between positive ends of two molecules.
The image above depicts water molecules. It results from the attractive force between a hydrogen atom covalently bonded to a very electronegative atom such as a N O or F atom and another very electronegative atom. A consequence of hydrogen bonding is that hydrogen bonds tend to arrange in a tetrahedron around each water molecule leading to the well-known crystal.
An attraction between the positive end of one molecule and the negative end of another molecule. Which statement describes a hydrogen bond between two water molecules. 4 Which answer below best describes the type of bond occurs between two oxygen atoms.
Each water molecule can form 2 hydrogen bonds between oxygen and the two hydrogen atoms in the molecule. 2 What is the bond between 2 oxygen atoms. Water is polar due its bent structure and an unequal sharing of electrons the slightly positive hydrogen atom is attracted to the slightly negative oxygen atom within a single water molecule.
Due to the electronegativity difference between the atom pairs mentioned electrons are unevenly shared across the covalent bond. Hydrogen bonding is a special type of dipole-dipole attraction between molecules not a covalent bond to a hydrogen atom. 3 What term describes the bond between atoms in a molecule of oxygen.
Hydrogen Bonding and Common Mistakes.

The Strong Polar Bond Between Water Molecules Creates Water Cohesion U S Geological Survey
Why Life Depends On Water Biology For Non Majors I

The Strong Polar Bond Between Water Molecules Creates Water Cohesion U S Geological Survey
Belum ada Komentar untuk "Which Statement Describes a Hydrogen Bond Between Two Water Molecules"
Posting Komentar